Blog 4: Week of 10/21/2018-10/27/2018: for this week, using lecture ten, I will discuss how can we potentially employ tissue engineering for bone regeneration using the bioactive biomaterials discussed.
The endoskeletal system is an essential system that facilitates movement, load-bearing role, and providing the structural maintenance and for the protection our most vital and delicate organs such as the brain and heart.
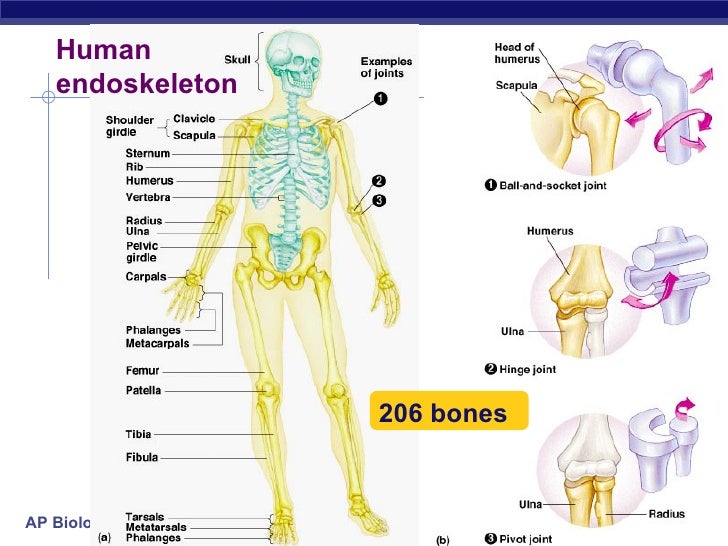
Figure 1: the human endoskeleton system consisted of 206 bone that provides structural support, organ protection, ion homeostasis, and site of immunological cell regeneration and maturation.
In addition to these primary structural supports, it serves a key function in Ca and P homeostasis, and function as the site of immunological cells generation (the bone marrow for hematocrit regeneration). The homeostasis of Ca and P play a significant role in signal activation or inactivation throughout the various muscle types in the bone. Consequently having a misfunction of a bone tissue induces huge complications in one life..causing one to lose one or more of the above inherent life functions of the bone tissue.
In order to alleviate these issues, biomaterials have been employed in different hallmarks. Nevertheless, the proposed implantation of these biomaterials in the past mostly has been in the form of a bio-inert material(1). And so, the contemporary consideration biomaterials to incorporate into tissue engineering methods, toward a design that employs bioactive materials that will integrate with biological cells for the regeneration of the bone tissue. Before addressing how tissue engineering could be employed for bone tissue regeneration, it’s relevant to discuss the fundamental organizations regarding the bone tissue.
The bone is made of bone matrix and the cells that made it. The bone matrix consists of osteoid, an organic material which includes various proteins and mainly collagen type I which dictates the tensile strength of the bone. The second constituent of bone matrix is hydroxyapatite, an inorganic mineral which comprises calcium phosphate crystals which dictates the rigidity and density of bones.
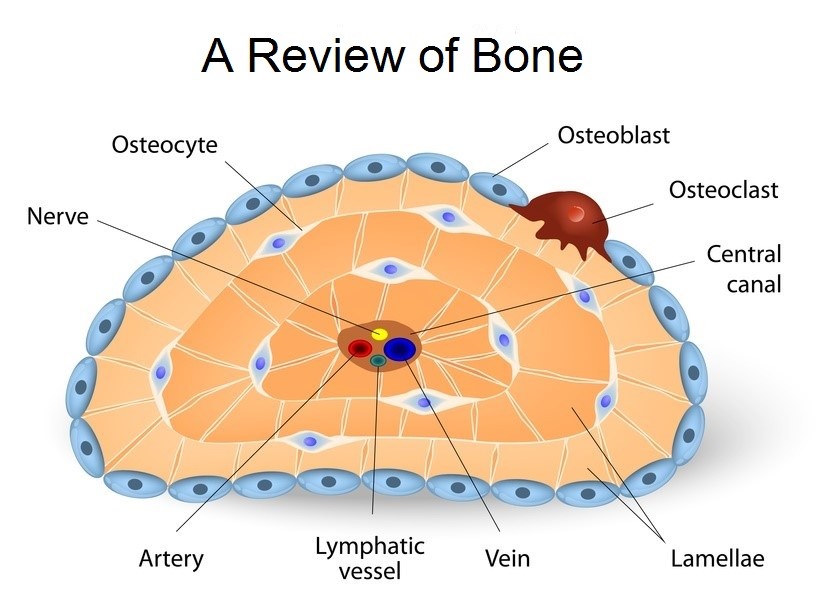
Figure 2: bone cell types that synthesis the bone matrix which serves as the framework for bone regeneration.
The cells that made matrix can be grouped into three main groups. The osteoprogenitor cells which are the precursor for the osteoblast cells which growth factors. The first types of cell that build the matrix are the osteoblast cells, which builds (synthesis) the two matrix components which are the primary structures for bone tissue regenerations. These osteoblast cells then matured towards osteocytes which occupy space in the matrix with sensors for bone cellular communication (are considered mechanosensory cells of the bone). And the last types of bone cells are osteoclasts that crash bones matrix to release Ca and P ions which play in reabsorption of bone.
The osteoblasts build a bone while the osteoclasts crash the bone. Thus the bone is under a constant remodeling homeostasis. The implication of the remodeling of the bone is that it keeps the homeostasis of Ca and P ions in the bone. When calcium is needed in the body for signal activation or inhibition, the osteoclasts crashes bone to release these ions and when the body has high Ca and P ions, the osteoblast take up these ions to build the bone.
So, knowing the physiological regeneration of bone tissue, how can we use biomaterials to regenerate bone tissues in order to substitute degraded bones? Given the high demand of clinical need of biomaterials for orthopedic, we can also tailor the biomaterials to be osteoinductive (able to support the differential of progenitor cells to an osteoblast lineage), osteoconductive (cable to promote bone growth and encourage the ingrowth encompassing bone) and finally osseointegration (cable of integrate within the surrounding bone) (2).
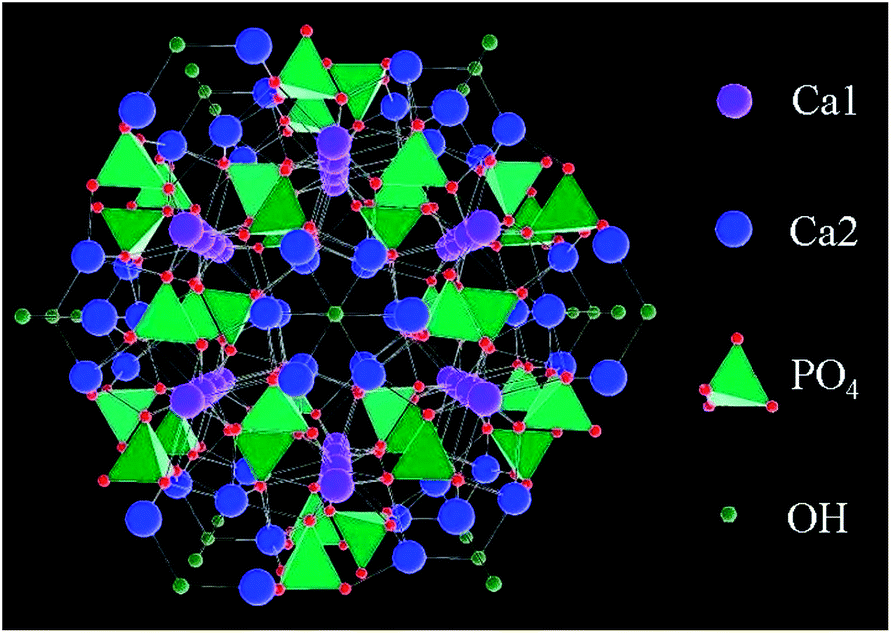
Figure 3: Hydroxyapatite, the major consist of the bone tissue matrix and a major candiate of biomaterirals in bone tissue engineering.
Therefore, biomaterials are required to be bioactive materials (including bioactive ceramics, bioactive glasses, and or a combination with polymers) to be incorporated in bone tissue engineering. Using these bioactive materials, the scaffold (framework) will be built in a way that over time, these materials will be biodegraded slowly and replaced with the patient’s own newly regenerated bone tissues.
Generally speaking, the bioactive inorganic materials that encompass tricalcium phosphate, bioglasses, and their combination with polymers can be tailored to design varies characteristics of a specific scaffold. Once scaffold is designed with these bioactive and biodegradable materials, various growth factors will be delivered to the scaffold using nanoparticle in order to enhance the regeneration of bone tissues as shown below (3).

Figure 4: different mechanism of introducing growth factors in the bone matrix to facilitate bone tissue regeneration
Finally, in modeling the bone tissue engineering, if we can master the modeling of biological complexity with three-dimensionally synthetics bioactive materials and the and closely mimic the physiological communication complexity of cells in the matrix, we can potentially expedite the field of bone tissue engineering to regenerate patient own bone tissue that will solve the incompatibility issue including immunological reactions due to the introduction of bio-incompatible materials in the body.
Sources:
- Lanza, Robert, Robert Langer, and Joseph P. Vacanti, eds. Principles of tissue engineering. Academic press, 2011.
- Stevens, Molly M. “Biomaterials for bone tissue engineering.” Materials today 11.5 (2008): 18-25.
- Lee, Soo-Hong, and Heungsoo Shin. “Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering.” Advanced drug delivery reviews 59.4-5 (2007): 339-359.