Blog 5: Week of 11/05/2018-11/10/2018: for this week, using lecture Twelve [Bulk Metalic Glass (BMGs)], I will discuss how can we potentially employ tissue engineering in blood glucose meter sensor and stent by increasing a surface area of biosensors or biomaterials for dynamic blood glucose level readout and robust blood vessel regeneration using BMGs.
Diabetes Mellitus is a global pandemic with over 388 million people affected worldwide (1). In addition to its direct consequences on patients daily life impediments, it’s also associated in doubling the risk of having a heart attack.
Type II diabetes accounts about 90% of all global diabetes cases and 80% of these cases are believed to be preventable by changing diet and lifestyle such as physical activities (2). And in order to accomplish these lifestyle goals, blood glucose meter (biosensors) play an important role.
However, the current glucose meter’s performance has been impeded with its biosensors ability in the body to interact with blood glucose. That’s despite designing an incredible device and inserting into the patient, the patient develops immunological reaction and prevents the glucose meter from performing its functions because the sensor will be blocked from interacting with blood glucose in flat sensor due to the biomaterials biochemistry interaction within the patient’s body.

Figure 2: Blood Glucose Meter. This type of blood glucose sensor requires multiple injections with the patient’s tissue which cause pains and distress. BMGs sensors, on the other hand, can be implemented in the patient’s body to measure dynamic blood glucose level, eliminating the need to poke a patient multiple times.
To further expand on this mechanism, let me discuss how the cells first interact with biomaterial inserted in tissue. One of the ways the cells react to biomaterials is that when biomaterials are introduced into a tissue in the body, proteins will have adhered into the biomaterial, then the macrophage interrogation will occur causing a big cell surrounding of the biomaterials. Eventually, the formation of envelope around the biomaterials increases inflammation and most importantly the biomaterials will have less integration (contact) with the surrounding cells which reduce the main purpose the biomaterials were inserted in the first place. You might ask, what is the problem with this process? Well, for instance, to dynamically measure the blood glucose level of a patient, a blood glucose meter (sensors on the glucose meter) needs to make a close contact with the surrounding cells so that the sensor will read out the blood glucose level. Further, the contact surface area needs to be large without increasing the device. Thus, to effectively monitor a blood glucose level, we have to overcome several obstacles from the biomaterial standpoint and from the immunological standpoint.
From biomaterials standpoint, how can we design the glucose sensor to have a more surface (contact) area while reducing its immunological inflammation (making it more biocompatible) without increasing its size? This is where the BMGs come in; by making a nanoscale division increment, it’s possible to achieve large surface while reducing immunological inflammations.

Figure 3: BMGs can be designed to have such large surface area in nanoscale which was hard to achieve such performance with other types of biomaterials. A large surface on biosensors enables to perform robust and dynamic blood glucose level readout.
BMGs has combined properties and functions between metals and plastics that enables it to achieve such promises. That’s plastics are easy to work with low temperatures but they’re brittle, and metals have the high mechanical strength yet they’re hard to work with at low temperature (challenging to process in a laboratory setting due to their grain boundaries). BMGs, on the other hand, can be used to make nanoscale biomaterials such as glucose meter sensors without being broken, which is very difficult to achieve such scales using ceramics and just metals because ceramics and metals have crystals (with boundaries of grains) and as the resolution gets smaller into nanoscale sensors, the grain boundaries in these materials (metals and ceramics) causes microscale mechanical failure (3). But the BMGs don’t have these grain boundaries, and so we can design nanoscale biosensors.
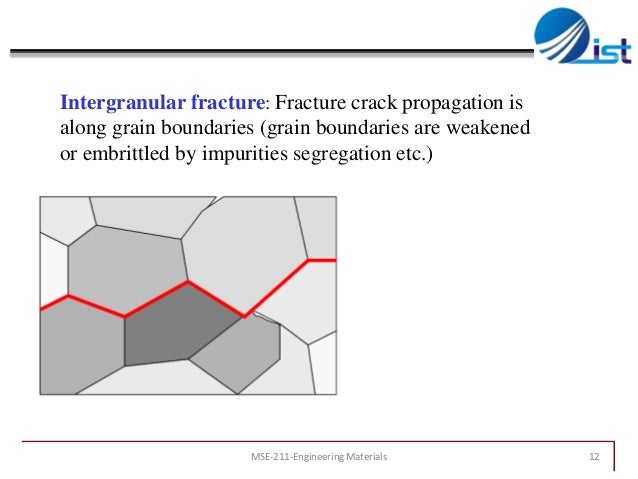
Figure 4: fracture crack in crystals due to grain boundaries which impedes ceramics refining abilities like BMGs. BMGs resolves fracture crack because they lack such big grain boundaries.
And the more surface area sensor has more contact with the blood sugar, outputting robust and accurate dynamic blood glucose level readout we can get. Therefore, BMGs enables to design and insert a glucose meter sensor into the patient’s body that can dynamically control the glucose level of a diabetes patient and encourages the patient to take immediate actions to better their health such as doing exercises.
The mechanical strength, easily processability, its corrosion resistance in combination with its biocompatibility, BMG is a promising biomaterial in designing and implementing tissue engineered stent for robust regeneration of blood vessels in the cardiovascular system. Particularly, porous BMG is promising for stent design and tissue engineered cells that can integrate and replace the biomaterials overtime.
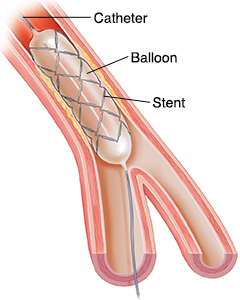
Figure 5: BMGs have promising abilities to be incorporated in stent design due to their mechanical strength, porosity (for cell integration & tissue regeneration) and corrosion resistance.
Moreover, BMGs have the mechanical strength of a metal to overcome a blood vessel shear stress without being corrosive and toxic for the surrounding cells.
Sources:
- (1) “Managing Diabetes Mellitus With Cell Therapy.” Villa Medica, 11 May 2018, villa-medica.com/cell-therapy-for-diabetes/.
- (2) “Physical Activity.” American Diabetes Association, www.diabetes.org/are-you-at-risk/lower-your-risk/activity.html.
- (3) Qazi, Meelu. “Chapter 8 Mechanical Failure.” LinkedIn SlideShare, 3 Dec. 2013, www.slideshare.net/MeeluQazi/chapter-8-mechanical-failure.

