Blog 6: Week of 11/26/2018-11/30/2018: for this week, using lecture fourteen [metals and their applications], I will discuss how can we potentially employ tissue engineering in bone and vascular remodeling using metals and techniques related in tunning mechanical properties.
Could Metals be integrated into Tissue Engineering? Before directly addressing the proposal, it crucial to present the current use of metals and issues associated with the various metal incorporation in the body. When it comes to mechanical strength, metals outperforms most materials that are available with a few exceptions and not to mention how they revolutionalize the construction industry. Wherever around us, most of the building incorporate some forms of metals mostly steel in structural support and related functions.

Figure 1: Osteoarthritis is a major issue in which structural supporting bone tissue such as cartilage is unable to perform their function due wearing out over time
Now the question becomes how can we take advantage of these mechanical strengths to integrate them for biomedical setting such structural support to mitigate problems in patients with osteoarthritis and related problem? Just to highlight some critical health problems that are related to structural support problem includes osteoarthritis, a condition in which the structural support and lubricant cells such as cartilages are unable to perform their physiological functions due to varies causes the main one being worn out over time.

Figure 2: Osteoarthritis is increasingly becoming a major issue as population ages
The prevalence of the disease increases as the aging population increases, just between 2013-15, over 54.4 million adults have been diagnosed with arthritis (1). Further, finding biocompatible metals with similar mechanical properties of bone such Mg is an active area of research for tissue engineering to solve cardiovascular problems such as atherosclerosis.
Metals have been used for structural support such as titanium alloy to alleviate these problems due to their mechanical strength. Despite their theoretical implications, when these metals introduce into the body, they cause unintended problems such activation of an inflammatory response.

Figure 3: bone is under constant remodeling to accommodate the change in patients body weight and to maintain blood Ca2+ and phosphate ions.
Above all, due to their mechanical strength, these metals have been mostly incorporated into structural support; a substitution for the bone. The problem is that the bone is constantly being remodeled in the body to compensate the patients by wait change. Further, the bone is the main source of Ca2+ and phosphate ion which are the two most essential ions in the body which involve in a single cascade mechanism including in the central nervous system, in muscle nerve connection and hormonal signaling cascade and much more (2). That’s when the body needs these ions, the bone will resorption via osteoclasts to release these ions into the bloodstream. When the patient’s weight increase, on the other hand, the osteoblasts bone cells will absorb these ions back into the bone matrix and make the bone dense or strong to support the body. But when the natural bone is substitute with a metal, these natural feedback system will be disturbed. The bone no longer remodels because the weight of the patient is shielded by the metal.
Furthermore, biomaterial reduction in the body another issue: metals causes an inflammatory response which can potentially lead to significant predicament to the surrounding tissue. Currently, researchers are working on how to take advantage of mechanical strength while reducing the above major issue including biocompatibility, that’s by tunning the properties of metals. Next, let’s highlight some of the breakthroughs in metallurgical modulation to that increase progress of tissue engineering.
The first issue of the metals is matching or closely resembling the mechanical properties such as the strength of the biomaterials in the body. Metals strength can be altered with a series mechanism to match the required specifications. For instance, if the metal we’re using has low compressive strength than the design specification, we can employ cold working process, that’s by heating the metal below its melting point and using different mechanisms such as rolling or drawing.
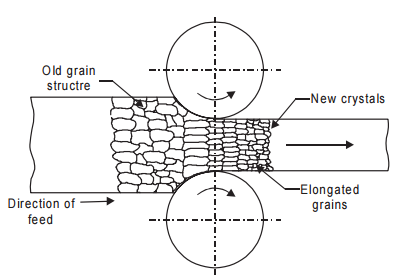
Figure 4: Cold working such as rolling enables reduce grain size thus limiting grain dislocation which enables to increase the strength of the metal
This mechanism potentially reduces the grain size of the given metal thus increasing boundaries between grains, and restraining the dislocation of the grains in the metals. Consequently, the metal strength will increase substantially. What if we want to reduce or correct the strength of the given metal to match again to specification? Again, we can use a countering mechanism: employ the annealing method, a heat treatment that reduces the mechanical strength at high temperature using three steps: recovery, recrystallization and grain growth. In short, the metal can be remelted and the rate of nucleation, the grain size will be controlled, thus controlling the strength of the metals to even with specifications.
The next problem is corrosion of the metal in the body. Here, you may suggest why not just use metals that have corrosion resistant and high mechanical strengths. Well, the environmental energy dictates what reaction happens in that environment: what I’m trying to say is that in the outside world where oxygen is abundant, iron has high reaction affinity with oxygen (due to the difference in reduction potentials), iron will corrode while aluminum seems not to corrode. But now we’re interested in integrating metals in the body, completely a different environment. The body is not at equilibrium rather it’s in steady state condition in which it’s constantly catalyzing redox reaction to maintaining the body at optimal conditions such as 37 degrees Celsius, PH of 7.4,…that means this new environment in the body changes the chemistry and can potentially corrode a metal easily which drives a material failure in the body (3). How can these problems be resolved? First, the standard reduction potential of each metal is carefully studied and metals with different characters will be selected to fit in that new environment. That’s some metals may improve to resist corrosion for body’s environment and yet they may not mechanical properties we’re looking like strength, thus we can take strong metals such as titanium alloys, and we can coat them with a metal with low surface chemistry within the body, that way we can eliminate the corrosion problem.

Figure 5: HA is associate in signal transduction for bone regeneration
Another issue is that biocompatible, that’s we want our metal based biomaterial not only just used as a structural support but want to also modulate the surrounding tissue to survive and perform its physiological function. This issue can be lessened by surface coating metals with bioactive materials such Hyaluronic acid (HA), which can potentially increase bioactivity such as osteointegration, a process that enables to integrate the sounding bone cells into inserted biomaterial so that these bone cells can perform their physiological functions, like growth, absorption of ions, resorption of ions (4). Further, metals such Magnesium is becoming an active area of research as they are biocompatible and has similar mechanical properties as the bone.
Putting all of these techniques together, we can potentially build biomaterials of composites of metals and other bioactive materials to potentially closely mimic the physiological function of the bone and other parts in the body. This domain is an active area of research to potentially transform tissue engineering, a branch of biomedical engineering that can operate with aim of substituting biological parts in tissue and organ level. That includes highly prevalent disease such as remodeling blood vessel, heart, kidney, pancreas and other tissue or organs by directly taking the patients to stem cells which make them their biocompatible and eliminating the need immunosuppress drugs.
Sources
- National Statistics | Data and Statistics | Arthritis | CDC. (n.d.). Retrieved from https://www.cdc.gov/arthritis/data_statistics/national-statistics.html
- Home. (n.d.). Retrieved from https://americanbonehealth.org/nutrition/how-the-body-maintains-calcium-levels/
- What Is Normal Body Temperature? (n.d.). Retrieved from https://www.webmd.com/first-aid/normal-body-temperature#1
- What is Hyaluronic Acid? How does Hyaluronic Acid benefit the body? (n.d.). Retrieved from https://www.hyalogic.com/about-ha/about-hyaluronic-acid/
